BGMA supply issues dashboard
Looking at April 2024[1], BGMA provides a monthly snapshot of the numbers of supply issues affecting generic[2] medicines in England, based on UK Government and NHS data. Since our analysis in February 2022, the Department of Health and Social Care has moved its supply issues update to being hosted on the Specialist Pharmacy Service (SPS) website. As a result, we have changed some of the ways we measure different kinds of supply issues to replicate the categorisations in the SPS medicines supply tool.
Supply issues as a proportion of presentations listed in the Drug Tariff[3]
Serious Shortage Protocols currently in place
- Number of generic presentations for which a Serious Shortage Protocol is currently in place = 1
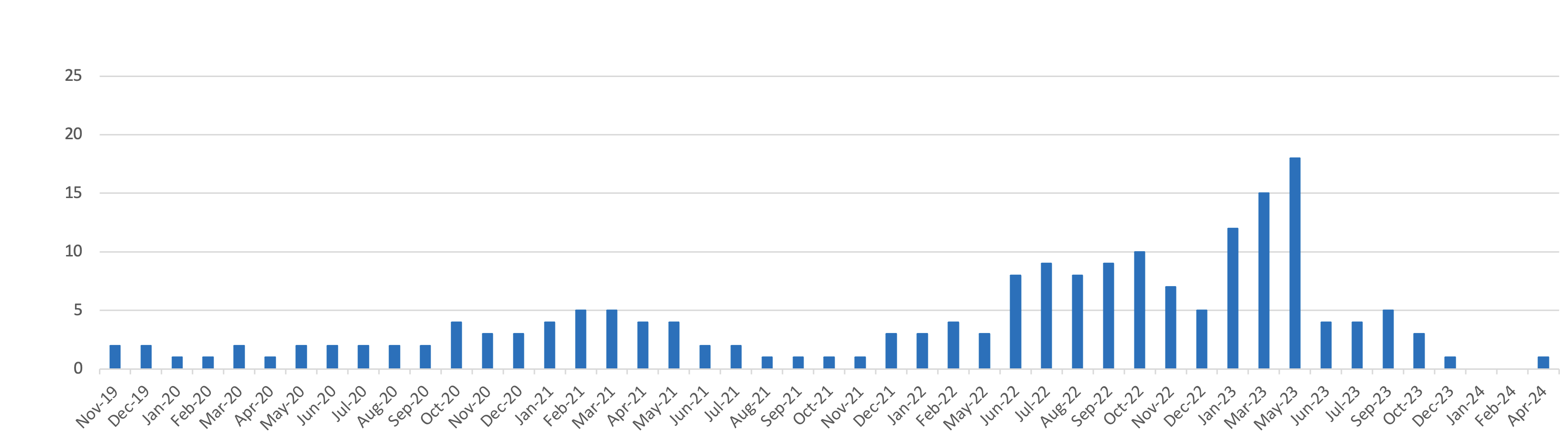
Medicines supply issues measured by Government and the NHS according to their patient impact
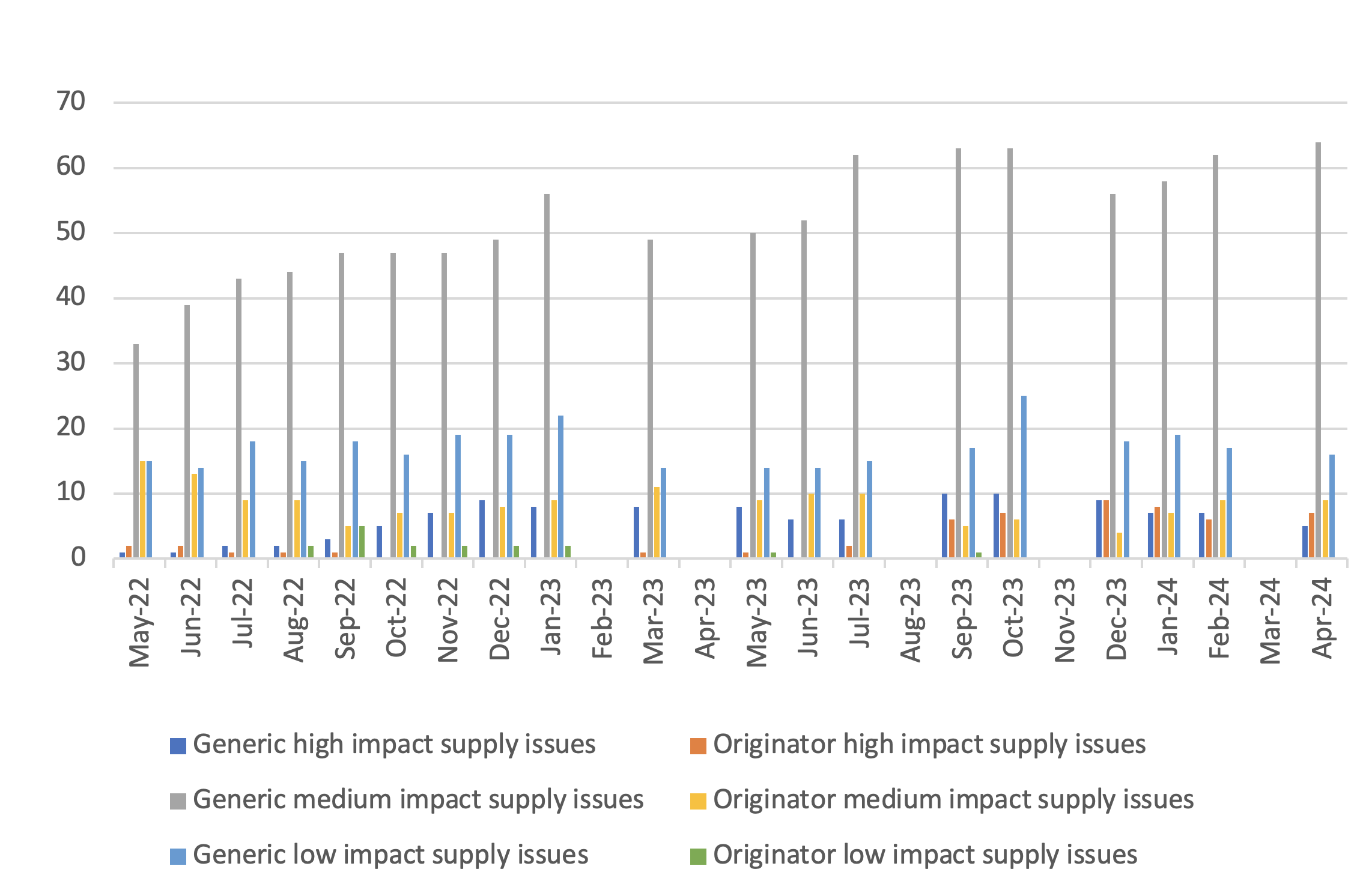
Number of supply issues and discontinuations identified in the SPS medicines supply tool
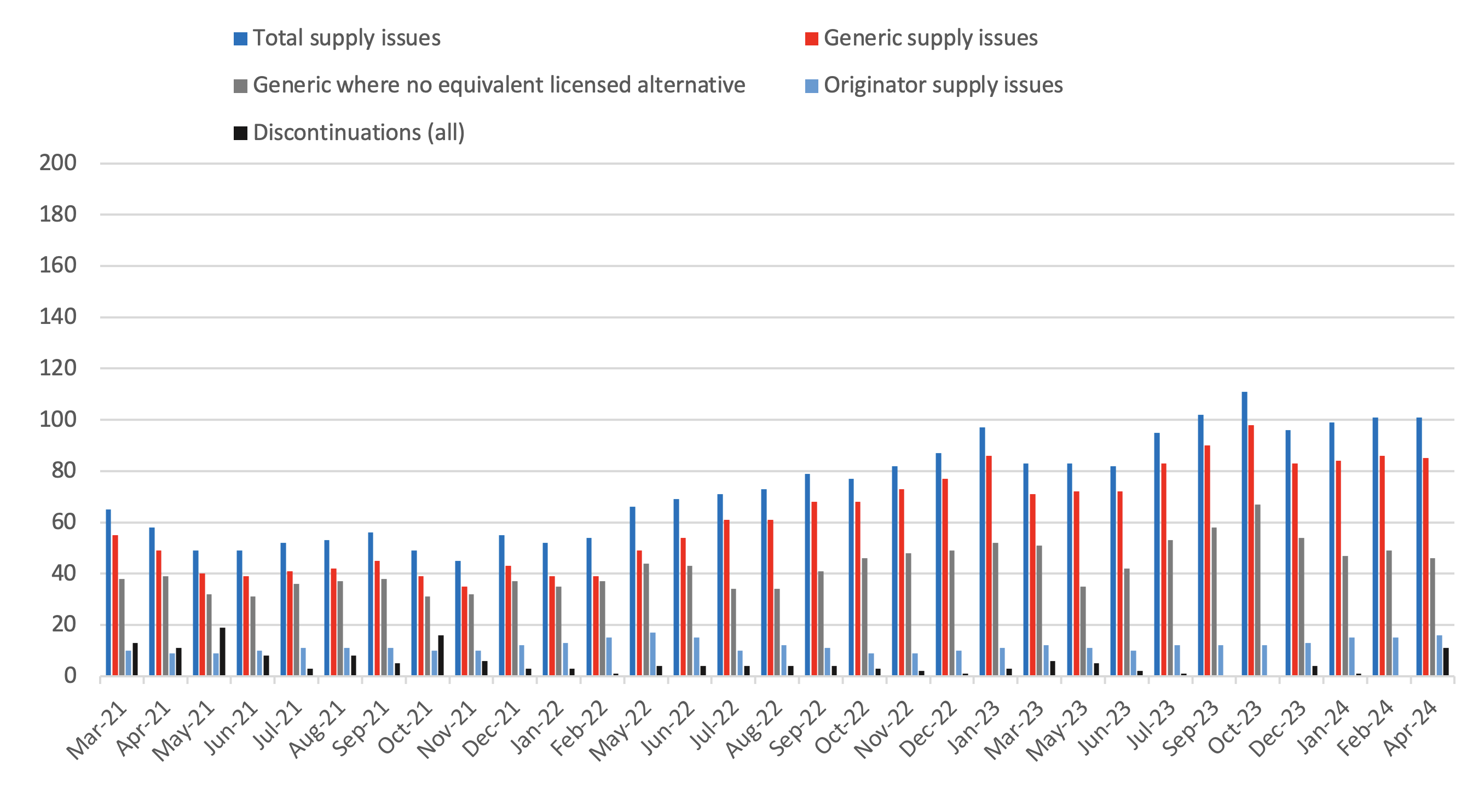
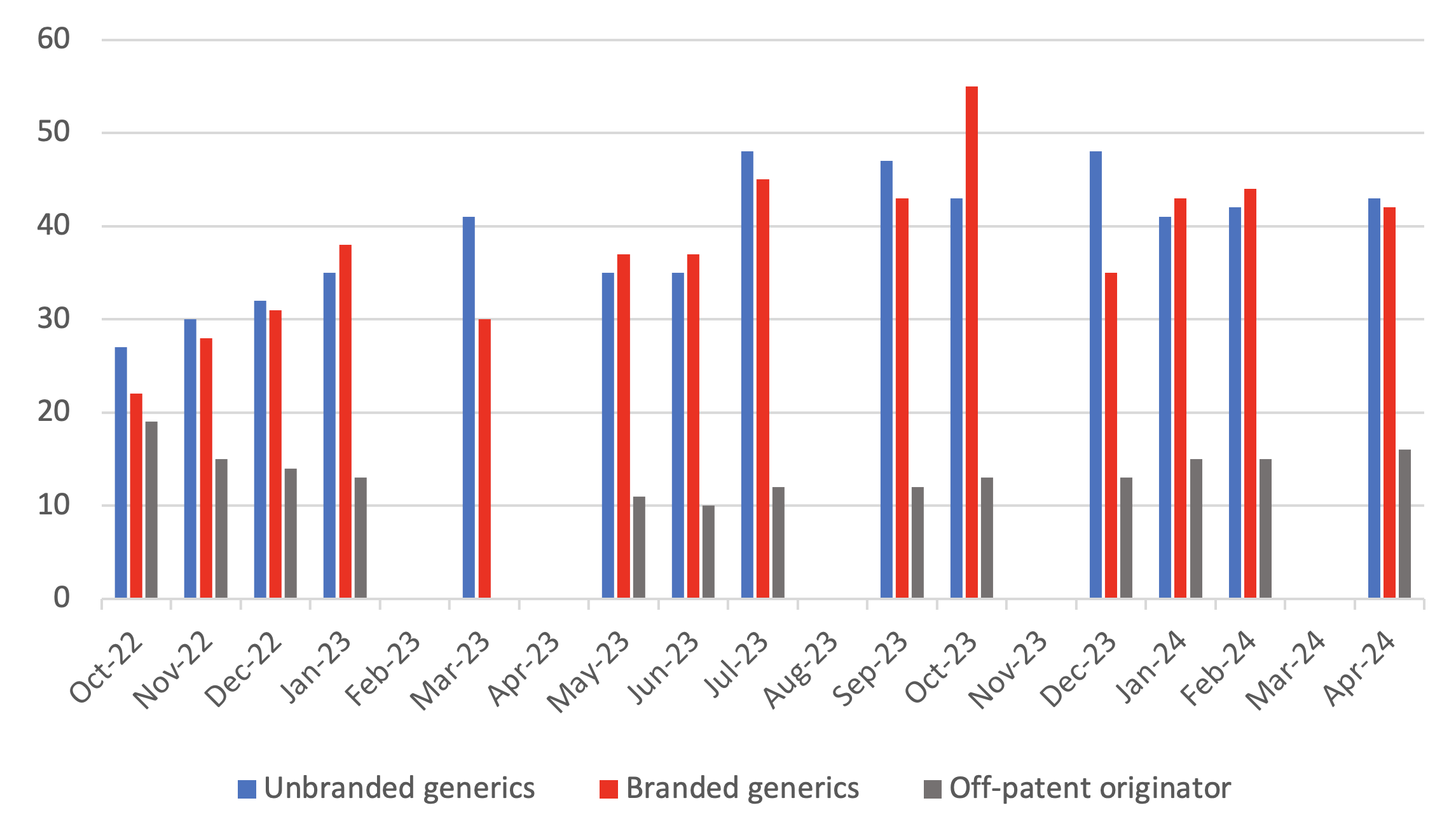
Breakdown of supply issues in off-patent market identified in the SPS medicines supply tool
Notes:
- 1 The BGMA’s analysis of the SPS medicines supply tool was conducted on 03 April 2024.
- 2 In this analysis, we define a generic medicine as a medicine that is not patent protected. As such, we include off-patent originator medicines as generic medicines, since they operate in genercised markets. The dashboard is BGMA’s interpretation of supply issues information issues by the UK Government and NHS. BGMA carries out this analysis categorisation on the basis of what is set out in the notes section.
- 3 Listed under Part VIIIA – the Basic Prices of Drugs. Source: https://www.nhsbsa.nhs.uk/pharmacies-gp-practices-and-appliance-contractors/drug-tariff/drug-tariff-part-viii. In April 2024, there were 3,819 different presentations listed in the Drug Tariff. From a sample, we found that 10% of presentations had the same molecule, strength and form as another presentation. We have therefore cut the Drug Tariff measure by 10% to 3,437 to ensure that we are accurately measuring against the supply issues identified in the DHSC supply issues update sheet (explained below), which is compiled on the same molecule, strength and form basis.
- 4 According to the NHS Business Services Authority: “An SSP enables community pharmacists, in the event of a serious shortage of any prescribed item that affects or may affect the whole or any part of the UK, to supply in accordance with the Protocol rather than supply in accordance with a prescription, without going back to the prescriber”. Source: https://www.nhsbsa.nhs.uk/pharmacies-gp-practices-and-appliance-contractors/serious-shortage-protocols-ssps
-
5The medicines are categorised by DHSC and the NHS according to impact tiers:
- Low impact: These supply issues are likely to carry low risk and management options and should result in patients being maintained on the same licensed medicine.
- Medium impact: These supply issues will require more intense manage options (such as using therapeutic alternatives, unlicensed imports or alternative strengths or formulations), which may carry a greater risk to patients/health providers than Tier 1 issues, but which are considered safe to be implemented at sub-regional level without further escalation.
- High impact: These supply issues will be more critical, with potential change in clinical practice or patient safety implications that require clinical or operational direction to the system. They will be expected to generate public and clinician concern. The response will be nationally coordinated and guided and the NHS may invoke its Emergency Preparedness Resilience and Response (EPRR) function.
- Critical impact: These supply issues will require additional support from outside the health system and will trigger the use of dedicated national NHS EPRR incident processes and procedures in order to provide additional support for the management of the shortage. Clear links and command and control mechanisms between the Medical Devices and Clinical Consumables Clinical Response Group, NHSE&I Central EU Exit Team, EPRR functions at both NHSE&I and ORC/DHSC, and Cabinet Office will be utilised.
- 6On a rolling basis, DHSC, with the help of the Commercial Medicines Unit covering secondary care, produces and updates a consolidated list of medicines with new, ongoing or resolved supply issues that are being actively managed to prevent or mitigate patient impact. The list is accessible on the SPS medicines supply tool to pharmacists and relevant NHS bodies that prescribe or oversee the prescribing of medicines. The medicines supply tool notes the level of availability of each medicine, providing advice to prescribers where needed. This also includes notice of medicines where the supplier has confirmed that it is discontinuing supply. Regional supply issues may not be captured, such as where other suppliers can fill demand.
- 7We have measured an equivalent licensed alternative as one where a pharmacist can make a switch to another licensed presentation without reverting to the prescriber to change the prescription. This might be where another manufacturer’s product or a different pack size is available, or a parallel import is available. Even where we have listed that there is no equivalent licensed alternative, a different form or strength of the medicine may be available which the patient can be safely prescribed.
- 8Where a generic is licensed and marketed by a brand name, either because it is a regulatory requirement from the medicines regulator, MHRA, or due to a commercial decision to differentiate the product from the supplier.
